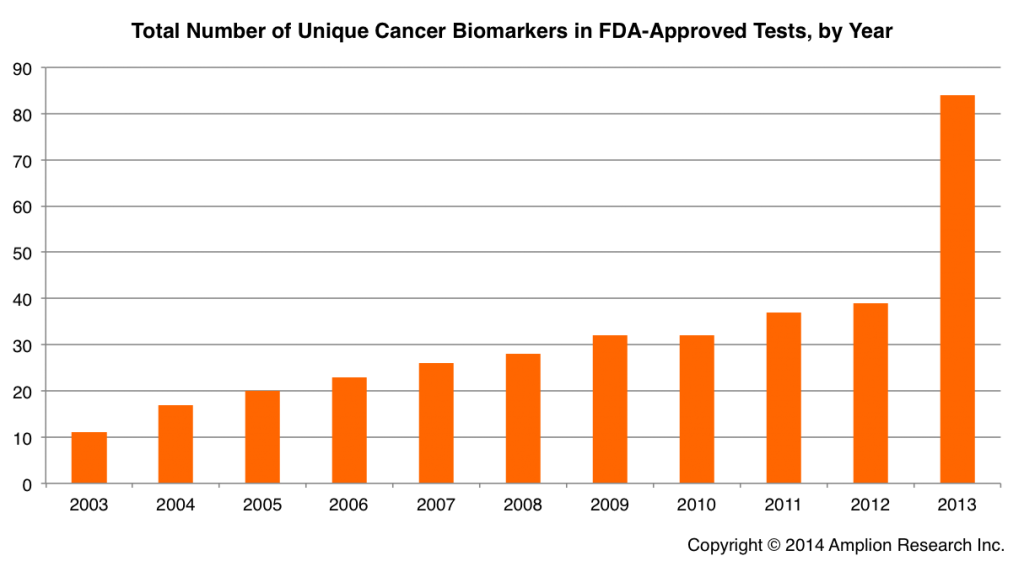
In a prior post we looked at publication trends for cancer biomarkers that are being used in more than one test, and in this post will look a bit closer at all 84 cancer biomarkers that have been approved by FDA, and the growth rate over the last decade.
2013 was obviously a banner year for new cancer biomarkers, as the release of theProsigna test by Nanostring introduced 45 new biomarker targets that had never before been used in an FDA-approved test, more than doubling the total number of approved biomarkers.
The annual growth rate in new FDA-approved biomarkers has been 23% over the last decade, and prior to 2013 it was 15%. No new cancer biomarkers have been approved by FDA so far in 2014.
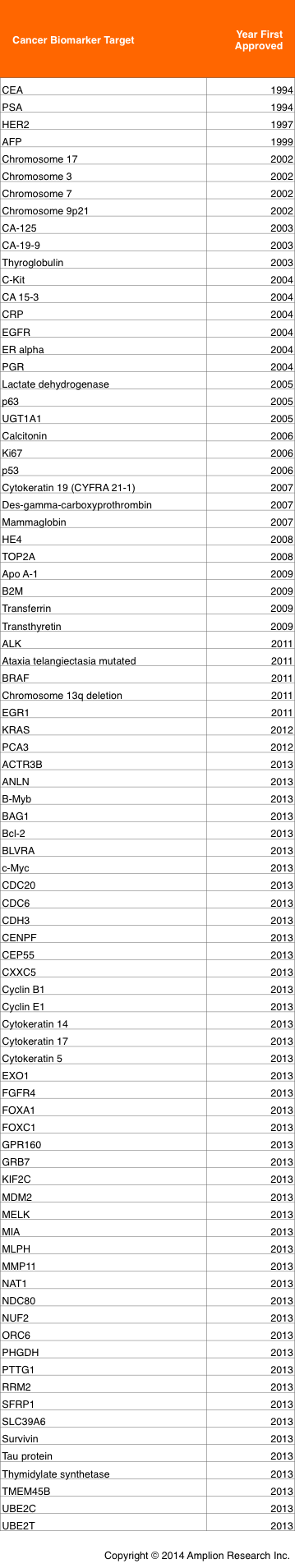
The table below lists every biomarker that has been approved by FDA, sorted by the year in which each was first approved.